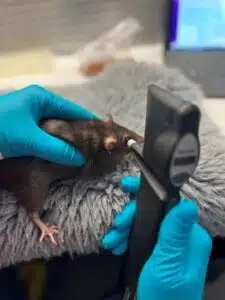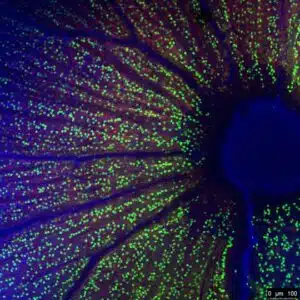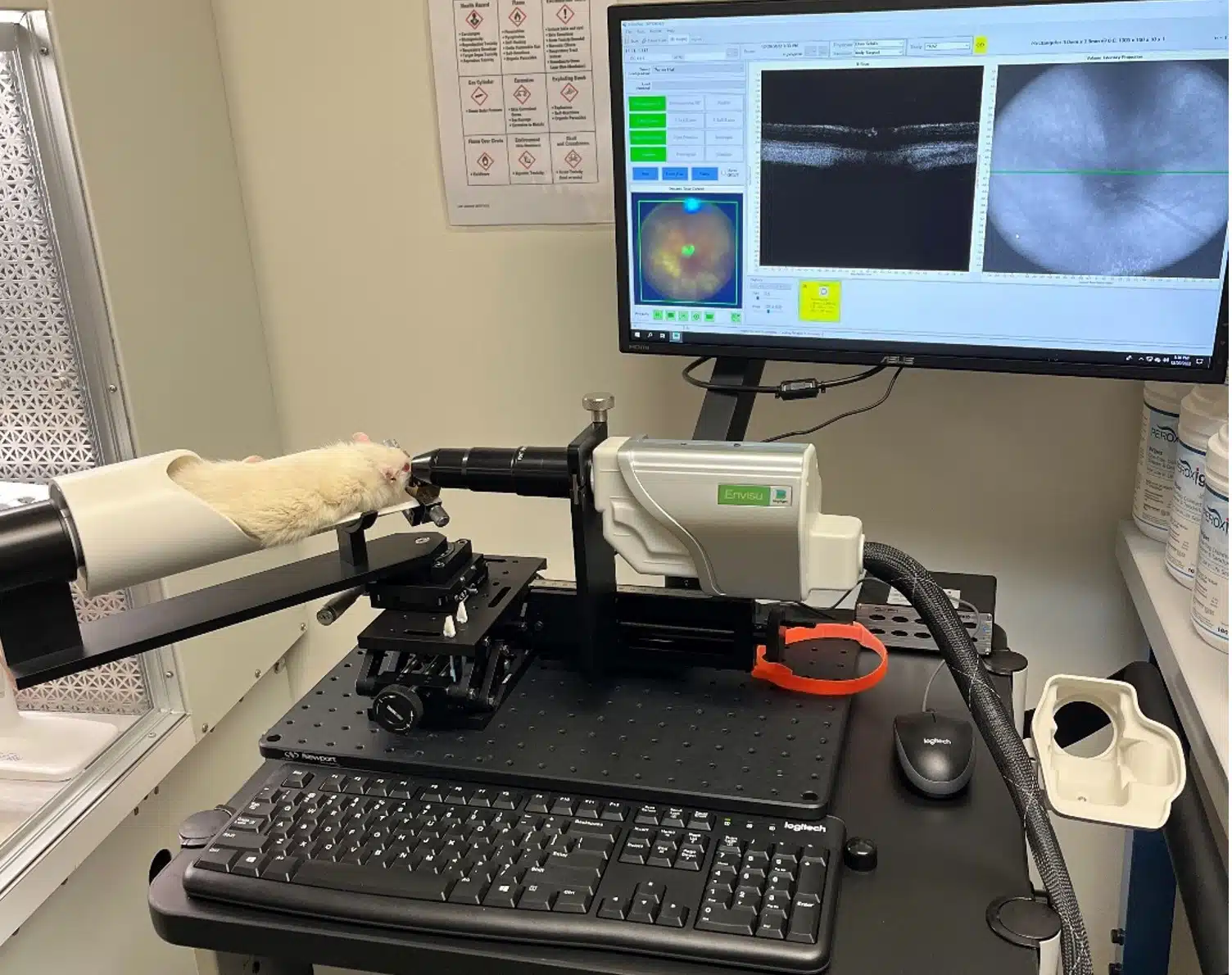
Spanning anterior to posterior disease models, we can assist! Our core business has been rooted in ophthalmic pharmacology for over 10-years. Our scientific staff is standing by to assist with developing your investigational product.
Ocular PK/PD Services
Perform ocular PK/PD studies in a variety of species spanning anterior or posterior applications.
Featured Capabilities
Our preclinical ophthalmic research team utilizes industry-best equipment to conduct studies. Quality equipment for quality results.
Your Results Await
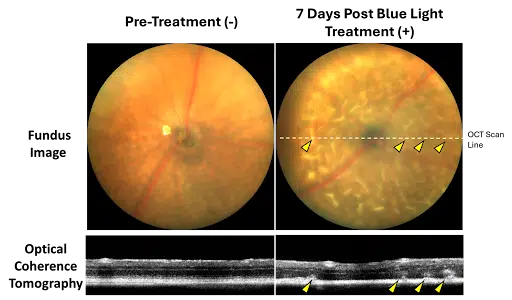
This featured Stargardt disease research model utilizes the ABCA4-/- transgenic knockout mouse for testing gene therapy and other investigational products for Stargardt disease and dry age-related macular degeneration. Blue light illumination in the ABCA4-/- strain activates reactive lipofuscin fluorophores and induces retinal lesions, which can be measured by fundus imaging, OCT, and histology. Functional impairment by ERG is also observed. This model is ideal for interventions aimed at directly or indirectly reducing lipofuscin burden or cytoprotective interventions intended to preserve RPE and photoreceptor structure and function.
Endpoints with Goals in Mind
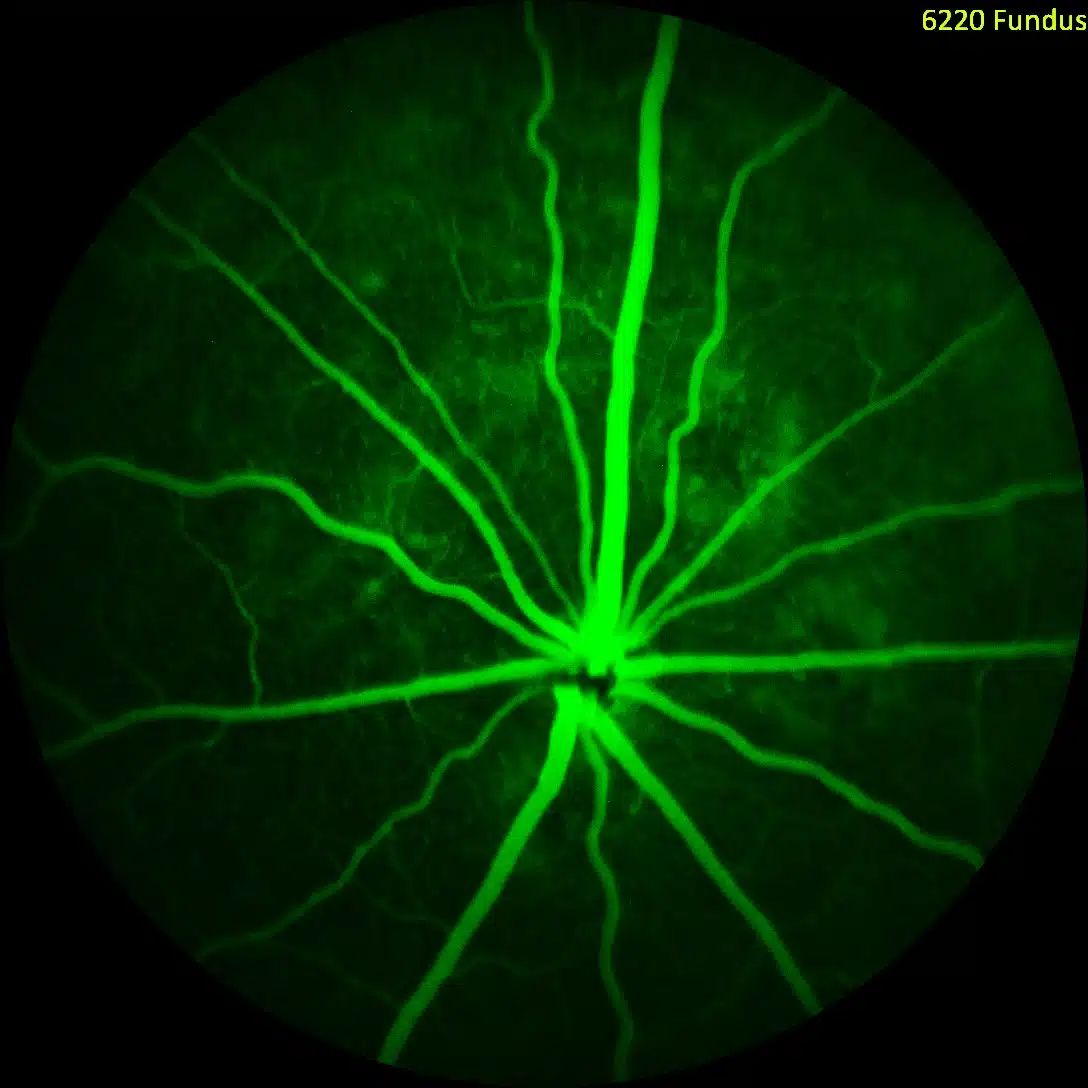
Advanced Imaging in CNV Model
We utilize state-of-the-art imaging modalities to provide the most robust data sets in support of client projects. If a picture is worth 1,000 words our data speaks volumes.
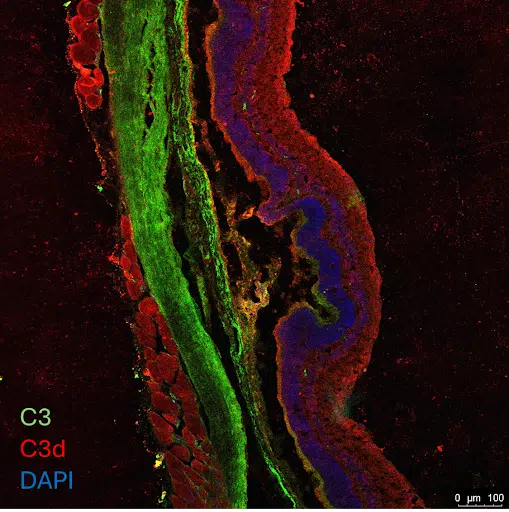
Histology for Target Engagement
We do not stop just at efficacy endpoints. Confirmation of target engagement and a clear understanding of mechanism of action inform downstream product development.
Capabilities and Services Summary
Frequently Asked Questions
Answers to some of the most common questions about preclinical ophthalmic research service offerings.