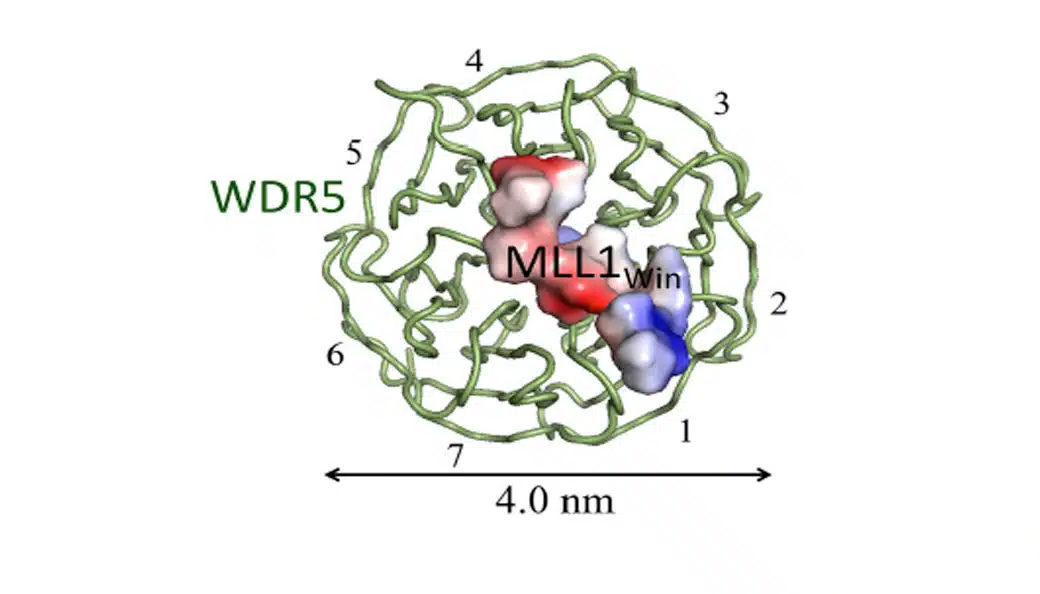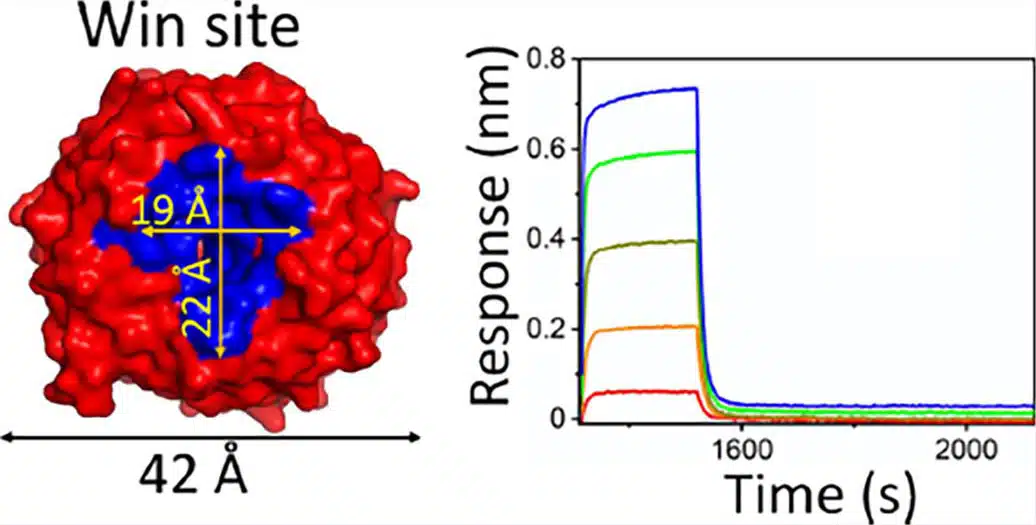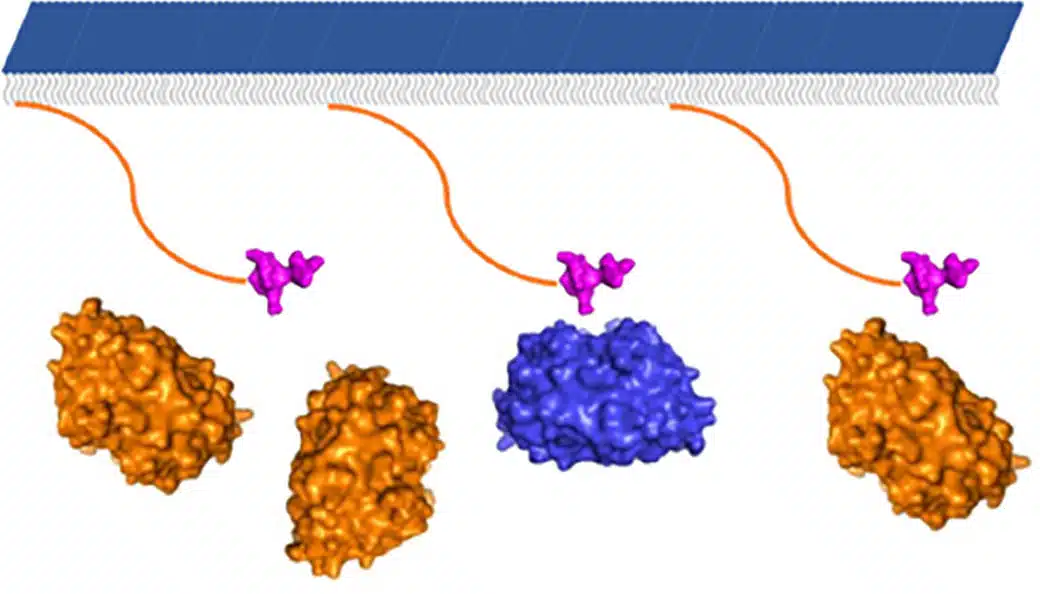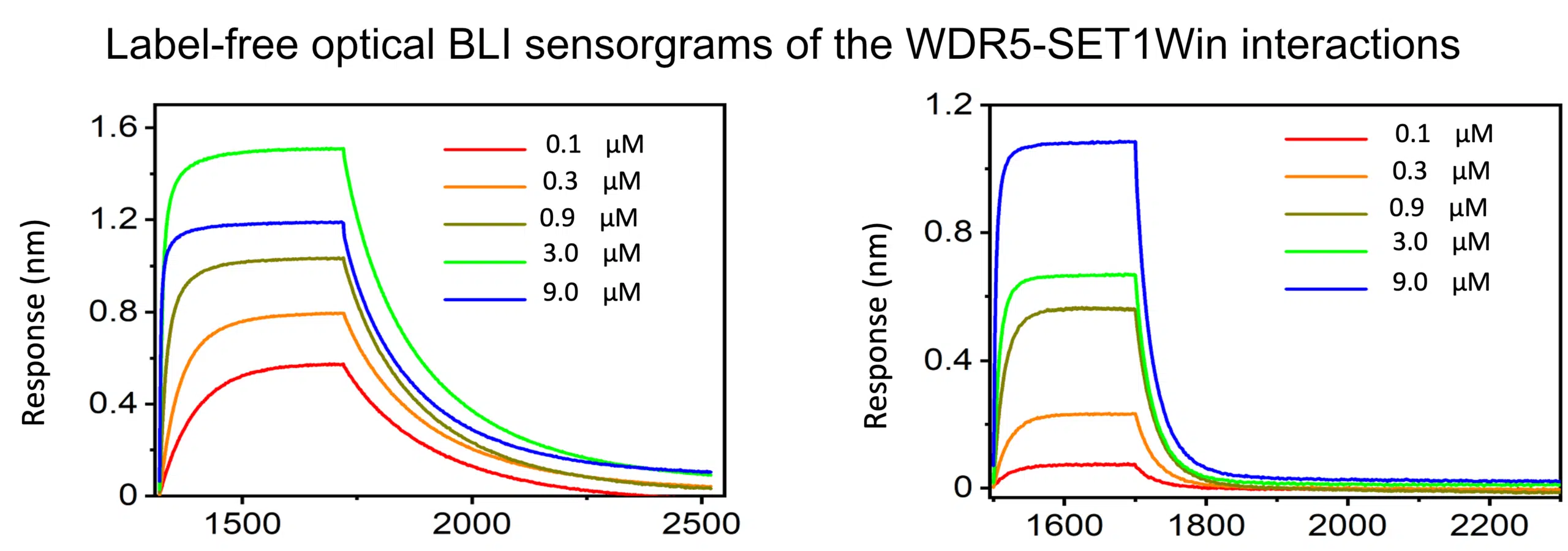
The Octet RED384 has 16 channel throughput and the sensitivity to generate publication quality quantitation and kinetics data.
What is BLI?
Similar to Surface Plasmon Resonance (SPR), Bio-Layer Interferometry (BLI) is a label-free, real-time, optical biosensor technique that measures the binding affinity and association/dissociation kinetics of biomolecular interactions.
The technique involves capturing one binding partner (the “ligand”) to the surface of biosensor tips that are coating in one of various tag capture systems, such as Ni-NTA (His-tag capture), Streptavidin (biotin capture), Protein A (Fc-tag capture), etc. The sensor tips are then dipped into solutions of analytes at various concentrations to detect and quantify binding. Association and dissociation data are collected, allowing for the time-resolved quantification of interactions and calculation of kinetic ka and kd constants, as well as binding affinity (KD = kd/ka).
BLI is an excellent technique for heavier analytes (>3-5 kDa) such as peptides and proteins, as well as analytes in complex matrices (e.g. serum samples) as there are no microfluidics that can become clogged. BLI is not a suitable technique for the direct measurement of small molecule binding interactions; for this analysis we strongly recommend our Biacore SPR analysis service.
Learn More About BLI:
BLI Publications:
We are Scientists & Innovators!