Uncategorized
Ichor Life Sciences Expands Ichor Clinical to Australia, Europe, and UK
LAFAYETTE, NY – January 24, 2024 – Ichor Life Sciences, a full-service, end-to-end contract research organization (CRO) and longevity biotechnology company, has set the stage for an exciting global advance in clinical trial services. Today, the company announced the expansion of Ichor Clinical’s services to Australia, Europe, and the United Kingdom. Pioneering a forward-thinking approach to enhance accessibility to international research and strategy, Ichor Life Sciences is ensuring that biotechnology and pharmaceutical companies have access to a world of possibilities. Lisa Sonneborn, MA, LMHC, President of Ichor Clinical, stated, “Our mission is to act as a global catalyst, offering personalized global clinical trial services, strategy, and regulatory guidance to life sciences companies of all sizes, including small and medium-sized companies that require a superior level of service.” Establishing collaborative processes and service offerings with concierge and boutique CROs in Australia, the UK, and Ireland, Ichor Clinical brings…
Read MoreIchor Life Sciences Announces Launch of Ichor Clinical Trial Services
LAFAYETTE, NY – November 14, 2023 – Ichor Life Sciences, a full-service contract research organization (CRO) and longevity biotechnology company, today announced the launch of Ichor Clinical Trial Services. With the founding of Ichor Clinical, the company is now able to serve biotechnology and pharmaceutical clients from early preclinical studies through late-stage clinical trials and FDA approval. Ichor Clinical offers a spectrum of customized clinical trial solutions, ranging from individual service options to full CRO assistance. Centered on solving each client’s unique challenges, the team specializes in critical areas such as protocol design, site identification and qualification, and recruitment and retention strategies. Ichor’s agile, relationship-focused approach allows the company to address complexities that may be difficult for larger, more standardized CROs, including assisting ongoing trials when they encounter enrollment hurdles or operational complications, restoring the trial’s trajectory toward success. Ichor’s preclinical offerings include protein services, pharmacology and discovery…
Read MoreIchor Publishes Article Unraveling Evolutionary Mechanisms of Phase Separation Proteins in Extreme Microorganisms
Lafayette, N.Y. – September 8, 2022 – Ichor Life Sciences, a premium preclinical research organization, recently published an article in collaboration with SUNY-Upstate Medical University. The article, “Coevolution of the Ess1-CTD axis in polar fungi suggests a role for phase separation in cold tolerance”, investigates the unique properties of proteins associated with the Ess1-CTD axis and their coevolutionary adaptation enabling tolerance to extreme conditions. Recent advancements have indicated that bimolecular condensates may facilitate a wide range of biochemical processes that allow organisms to maintain dynamic control of cellular processes in unfavorable conditions. The article is quoted, “On the basis of these findings, we propose that localized sequence divergence within the CTD, and potentially in the intrinsically disordered regions (IDRs) of other proteins, may be an evolutionary driver to enable adaptation of organisms to extreme environments by altering biophysical properties such as the ability to undergo phase separation…Our findings lay the…
Read MoreLento Bio Raises $680,000 in Oversubscribed Seed Round Led by Ichor Life Sciences
Potsdam, N.Y. – September 6, 2022 – Lento Bio, Inc., a preclinical pharmaceutical company developing small-molecule therapeutics to restore ocular lens flexibility and near-vision in presbyopia patients, announced today they have raised $680,000 in funding during their recent oversubscribed seed round. Ichor Life Sciences is the lead investor for the round, providing $400,000 in funding to Lento Bio. Lento Bio was launched in June of 2022 and is an Ichor Life Sciences portfolio company. The seed round will be used to conduct research and testing that will establish proof of concept for Lento Bio’s therapeutics and help optimize lead compounds in early-stage pre-clinical studies. “We are extremely grateful for the support for our science that Ichor and other investors have shown us through this funding round”, said Dr. Kris Barnes, PhD, Lento Bio CEO. “Since our launch, our focus has been to develop small-molecule therapeutics to treat presbyopia. This funding…
Read MoreIchor Life Sciences Invests $1.5 Million in Mitochem Therapeutics for Ocular Aging Disease Research
LaFayette, N.Y. – August 11, 2022 – Ichor Life Sciences, a preclinical contract research organization, today announced a $1.5 million investment in MitoChem Therapeutics. Based in Charleston, South Carolina, MitoChem Therapeutics develops treatments to address the effects of mitochondrial dysfunction—a hallmark of aging—in neurodegenerative diseases. MitoChem Therapeutics and Ichor Life Sciences will work together to advance the development of a small molecule pharmaceutical eyedrop, targeting the mitochondria to treat age-related eye disease. “Our lead compound is unique in its ability to treat a key mechanism of disease in a broad range of hard-to-treat diseases,” said Michael Voevodsky, CEO of MitoChem Therapeutics. “Ichor Life Science’s investment and capabilities will help advance our compounds into the clinic.” MitoChem Therapeutics is the third company focusing on treatments for ocular disease in the Ichor portfolio, along with Lento Bio, Inc. and Lysoclear, Inc. Ichor has significantly expanded its ocular capabilities under the leadership of…
Read MoreLento Bio, Inc. Appoints Two Ichor Life Sciences Executives as a Member of the Board of Directors and Scientific Advisors
Potsdam, N.Y. – Lento Bio, Inc., a preclinical pharmaceutical company developing therapeutics to target molecular damage driving age-related disease, announced today it appointed Dr. Kelsey Moody, PhD, MBA, CEO, and founder of Ichor Life Sciences as a member of its board of directors. Lento Bio also appointed Dr. Moody and Dr. Aaron Wolfe, PhD and chief science officer of Ichor Life Sciences, as scientific advisors. Lentio Bio was launched early this year. The company is focusing initially on developing pharmaceutical eyedrops to treat a common vision disorder, presbyopia, or age-related farsightedness. Dr. Moody brings deep expertise in Lento Bio’s two primary areas of research, ophthalmology drug development and aging biology. Through his work with Lysoclear, an Ichor Life Sciences portfolio company, Dr. Moody also has experience developing therapies to treat age-related macular degeneration and Stargardt’s disease, which collectively affect over 200 million people across the globe. Dr. Moody also brings…
Read MoreIchor Life Sciences Expands Ophthalmology Capabilities with Christopher Schillo’s Appointment as Vivarium Director
LaFayette, N.Y. – Ichor Life Sciences, a premium pre-clinical contract research organization offering services in discovery through pharmacology, announces the hiring of Christopher Schillo as its new vivarium director to expand the company’s ophthalmology capabilities and capacity. Schillo is responsible for overseeing all operations within the vivarium, which includes managing staff, animal husbandry, and research operations as well as acting as Ichor’s Institutional Animal Care and Use Committee (IACUC) Chair, a requirement for all vivarium’s that house USDA species. Prior to joining Ichor, Schillo served as the Director of Preclinical Operations for Ora, Inc., a leading provider of eye research for pharmaceutical companies, where he oversaw pre-clinical operations and provided expertise in ocular drug development. At Ichor, Schillo will augment Ichor’s current offerings, including ophthalmology, oncology, geriatrics and more. “During his time at Ora, Chris proved his expertise in ophthalmology and ability to manage a world-class vivarium,” says Dr. Kelsey…
Read MoreLento Bio, an Ichor Life Sciences Portfolio Company, Launches with Aims to Develop Anti-Glycation Drugs for Presbyopia and Diseases of Aging
Potsdam, N.Y. –. Lento Bio, Inc., a preclinical pharmaceutical company focused on developing small-molecule therapeutics to target molecular damage driving age-related disease, announced its launch today. The company will initially focus on developing pharmaceutical eyedrops to treat a common vision disorder, presbyopia, or age-related farsightedness. Lento Bio will be supported and incubated by Ichor Life Sciences, a pre-clinical contract research organization, at Clarkson University’s Peyton Hall Biotechnology Incubator. Lento Bio is the first start-up company to join Ichor in establishing a North Country biotechnology cluster. Presbyopia is caused by stiffening of the eye lens, which stems from molecular crosslinks including advanced glycation end products (AGE) that cause tissue rigidity. The small molecule drugs being developed by Lento Bio will target underlying molecular damage accumulation with the goal of reversing the process of tissue-stiffening in the ocular lens. Upon successful completion of its first project, Lento Bio plans to apply its…
Read MoreIchor Life Sciences Portfolio Company, Auctus Biologics, Awarded NSF Grant to Develop New SPR Chip Technology
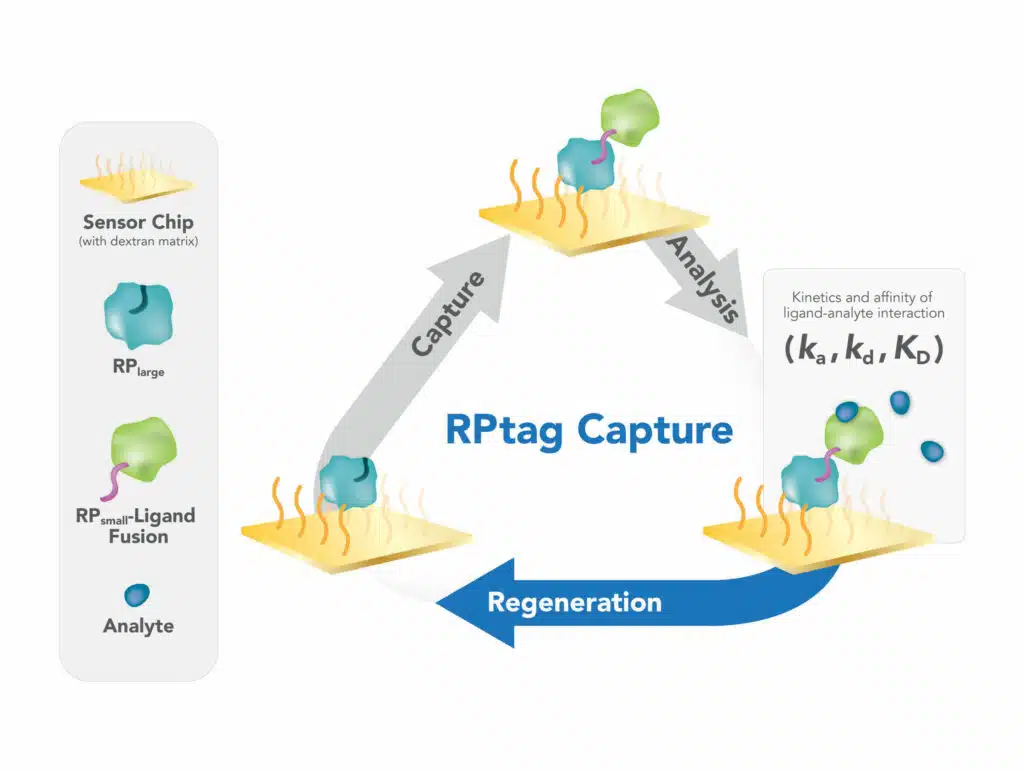
LaFayette, N.Y. – Ichor Life Sciences, a premium pre-clinical contract research organization offering services in discovery through pharmacology, announces that portfolio company Auctus Biologics has been awarded a Phase I National Science Foundation Small Business Innovation Research grant (award # 2129469) for $228,697.00. The grant will fund development of a new optical biosensor chip technology for use in the biophysical technique of Surface Plasmon Resonance (SPR). The gold standard for studying biomolecular interactions in the pharmaceutical and biotechnology industries, SPR is an essential method for characterizing the interactions between small molecule drugs and their protein targets. The proprietary chip technology created by Auctus Biologics will overcome key barriers in current state-of-the-art SPR technology, streamlining workflows, and accelerating the pace of drug screening campaigns. Auctus Biologics is a biotechnology company that develops antibody mimetics for therapeutic and diagnostic applications. “In keeping with Ichor’s core mission of pushing the R&D envelope, Auctus…
Read MoreClarkson University Features Professor Aaron Wolfe for Publication in Leukemia Diagnostics
In a post from Clarkson University, Ichor CSO Aaron Wolfe is featured for his recent publication in Nature Communications on WDR5 protein nanopore technology that has applications in diagnostics. Dr. Wolfe is quoted in the post saying “The outcomes of this research demonstrate the feasibility of single-molecule detection of protein analytes in a modular way..his research clearly allows us to envision a tool at the forefront of protein-based diagnostics for cancers and other diseases. We at Ichor feel that this collaboration is a strong testament to what is possible when the barriers of academic and industrial sciences are reduced” Clarkson University responded in their news release: “The paper is currently in the 99th percentile (ranked first) of nine tracked articles published at the same time in Nature Communications.” Ichor Life Sciences is an industry partner with Clarkson University and has built facilities at the university to establish a biotechnology cluster…
Read More