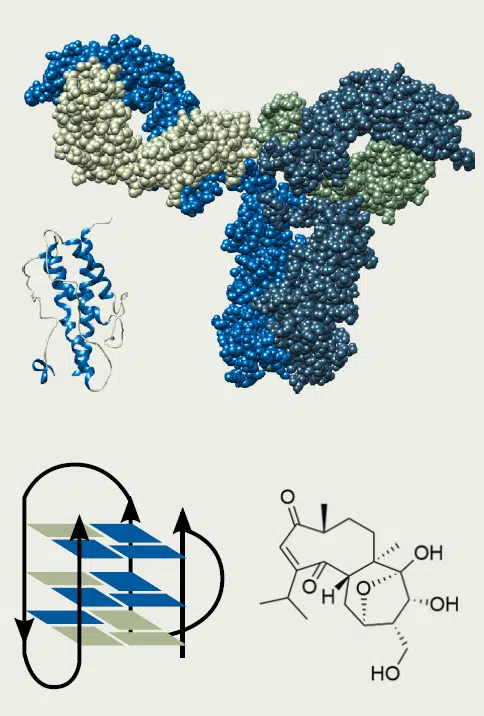
Circular Dichroism (CD) is a biophysical technique used to characterize the higher order structure and conformational stability of biological samples, including proteins & peptides, nucleic acids, and small molecules.
CD is the difference in absorbance between left and right circularly polarized light by a chiral molecule that contains a chromophore. The technique lends itself to many applications, including quantifying stability, elucidating the secondary and tertiary structures of proteins, and conducting robust statistical comparisons of higher order structural changes in biotherapeutics.
Secondary Structure
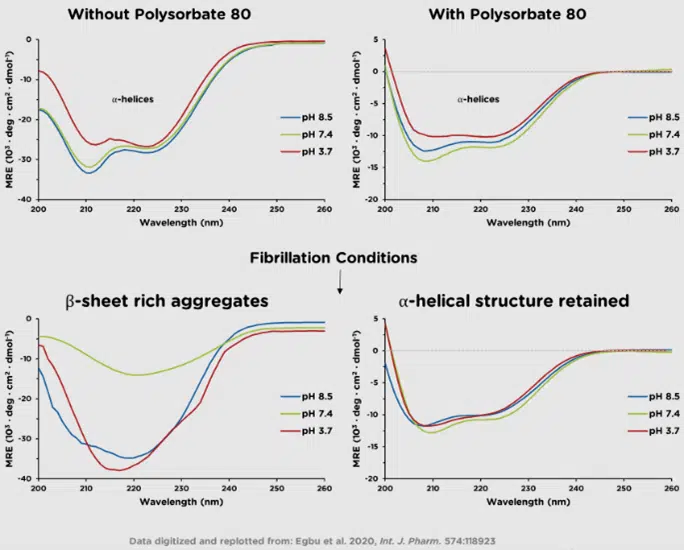
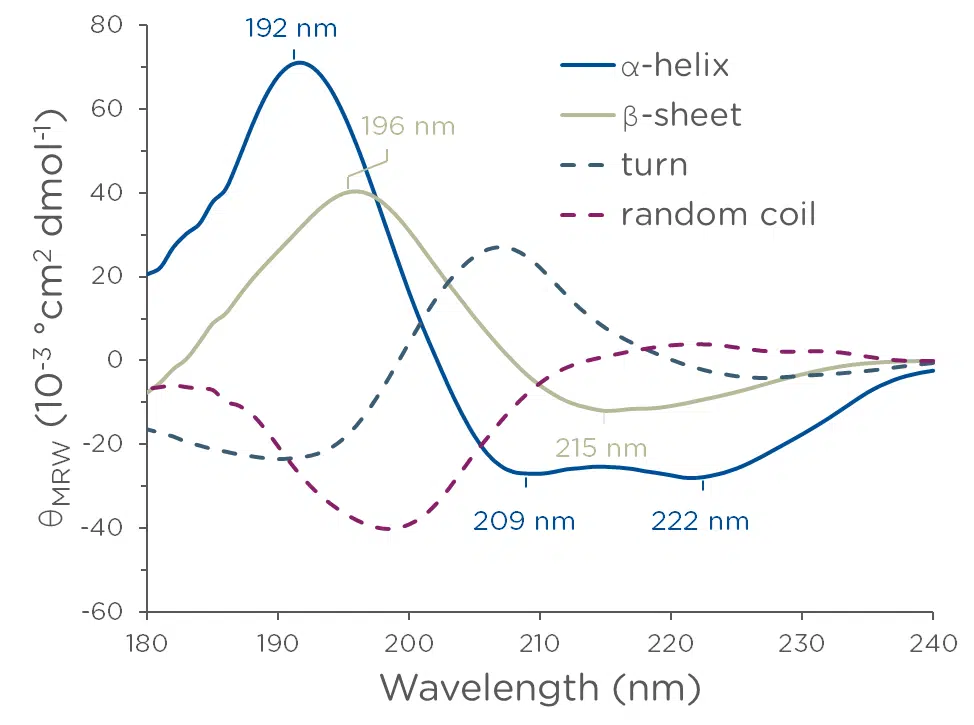
Far-UV CD (260-180 nm) reports on secondary structure motifs, such as α-helices and β-sheets. Structural elements are readily quantifiable with commercially available software such as ProtaCAL.
Tertiary Structure
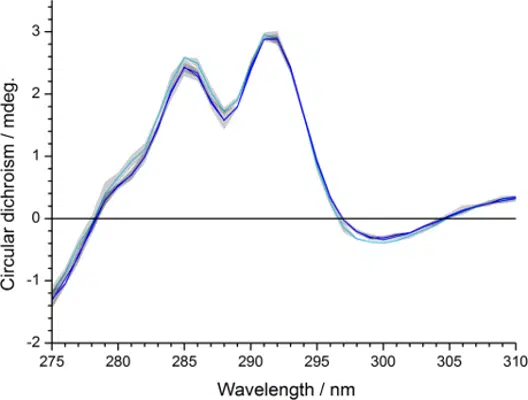
Near-UV CD (350-250 nm) reports on elements of tertiary structure, such as the orientation, solvent exposure, and overall fold of Trp, Tyr, & Phe residues, as well as disulfide bonds.
Reliably measure protein Tm values
CD measures protein conformational stability via thermal denaturation temperature ramps, yielding Tonset, Tm, van’t Hoff enthalpy, & ΔG.
HOS & Quality Control
Thanks to the robust automation in our Chirascan Q100 system, we can conduct statistically meaningful higher order structure comparisons on different batches of proteins or biotherapeutics.
Critical Data for Biotherapeutic Development
CD spectroscopy is now recognized as a crucial part of the biophysical characterization process where HOS comparisons are essential for defining critical quality attributes of biotherapeutics and strengthening the totality of evidence for regulatory submission.
Label-Free
CD utilizes the intrinsic chromophores present in most biomolecules, meaning there’s no need for labeling or adding dyes.
High-Throughput
We can run full CD characterization and referencing on up to 96 samples in a single automated experiment with extremely high precision.
Low & Nondestructive Sample Requirements
Depending on the experiment, CD can be run with protein concentrations as low as 0.1 mg/mL. Sample can be recovered and returned to you.
Formulation Screening
CD is a powerful screening tool to quickly determine the appropriate buffer, pH, ionic strength, and excipients needed to keep your protein happy and properly folded.
Proteins & Peptides
Proteins and peptides are the most well-studied CD substrates. Distinguish not only α-helix & β-sheet, but also 310-helix, π-helix, random coils, turns, and so much more.
Nucleic Acids
Nucleic acid structural motifs, including different G-quadruplex topologies, can be easily distinguished.
Small Molecules
CD is an indispensable technique for characterizing chiral small molecules, including natural products with unknown stereochemistry.
What sets Ichor’s CD services apart?
Ichor’s CD services include sample handling, standard assay execution, data analysis & reporting, and development of custom workflows for unique projects. No project is too big or small.
Higher Order Structure Analysis (HOS)
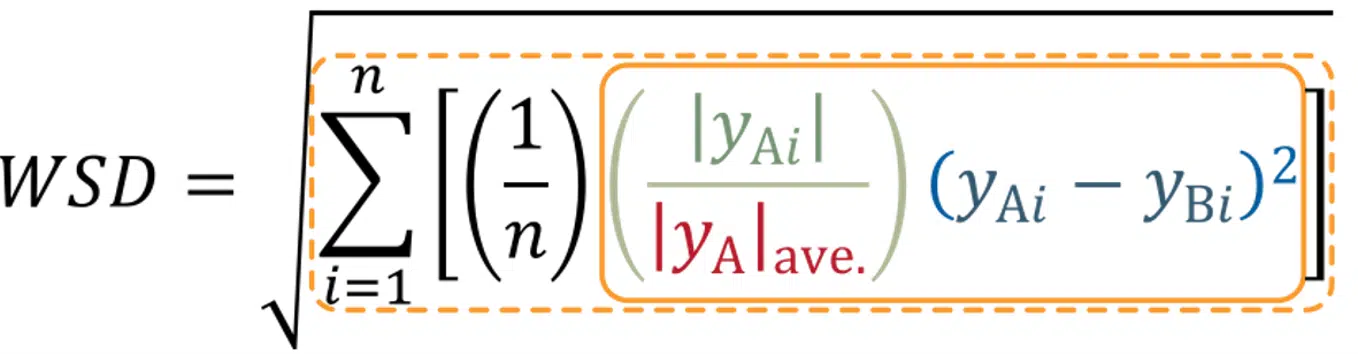
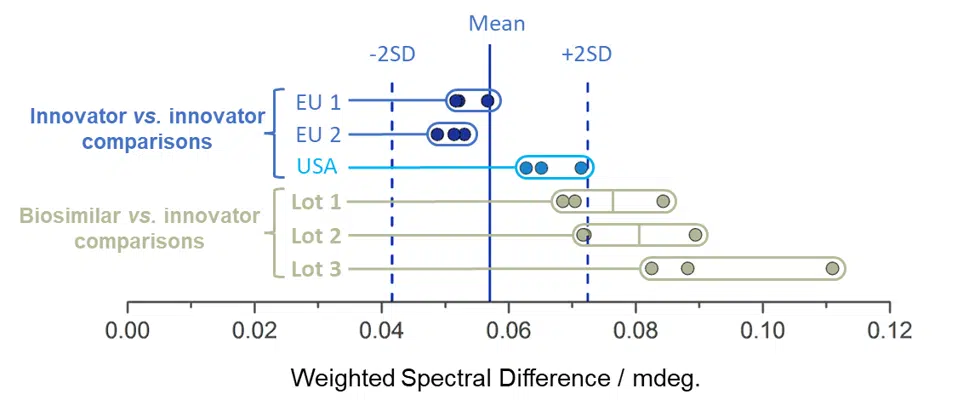

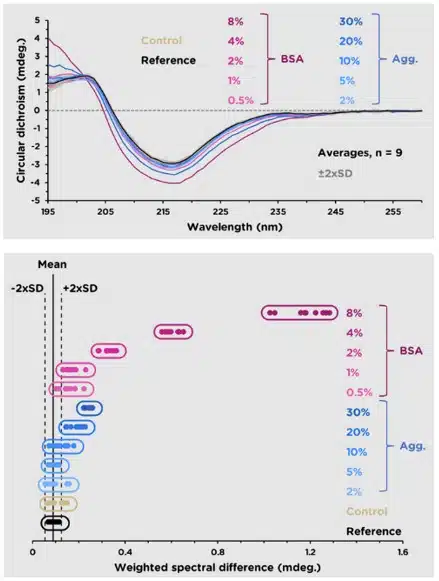
(Three images – 1) spectral overlays of multiple sample lots with error in gray, 2) WSD equation, and 3) WSD plot showing sample distribution about a mean +/- 2 SD.

Ichor’s CD services go beyond routine formulation screening experiments and into the realm of statistically powerful secondary and tertiary structure comparisons (often referred to as Higher Order Structure, or HOS). Comparison of spectra is conducted via the Weighted Spectral Difference (WSD) method. The resulting values and their standard deviations can be used as an objective measure of their similarity, which allows the data to be subjected to a quality range test and be included as part of the product’s Critical Quality Attributes.
CD is indispensable in biosimilar development:
“Circular dichroism" has been used/proposed in 96% of biosimilar applications involving mAbs and other biotherapeutics.”
–Regulatory consideration for characterization of HOS in biotechnology products, M. T. Gutierrez Lugo, Ph. D., OBP/CDER/FDA. 5th International Symposium on HOS of Protein Therapeutics 2016.
As the biopharmaceutical industry faces increasing demands for objective, statistically validated data in regulatory submissions, state-of-the-art CD spectrometry enables detection of minor changes in HOS and evaluation of their statistical significance early in the development process.
Examples include comparisons of innovator versus biosimilar lots (above) and forced degradation studies, which are used to investigate stability, storage conditions, and expected shelf life.
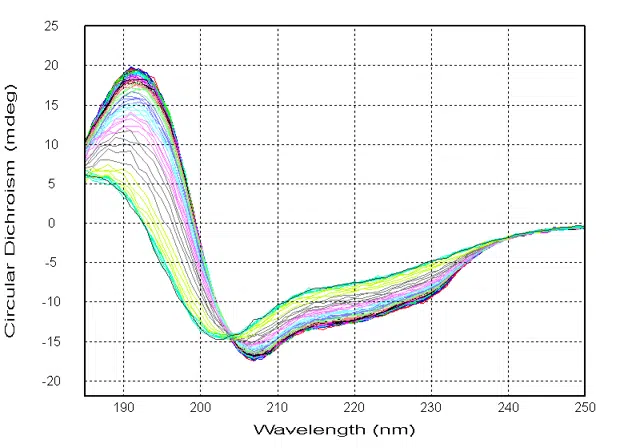
Overlay of CD vs. wavelength temperature isotherms.
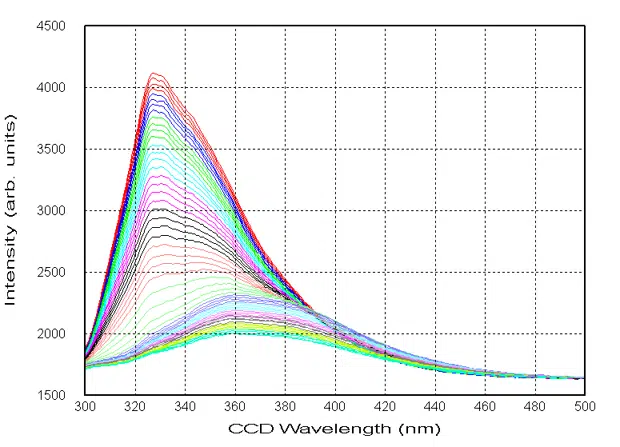
Overlay of fluorescence emission vs. wavelength temperature isotherms.

Thermal Denaturation – Temperature Ramp Experiments
All Chirascan systems can perform continuous, multi-wavelength temperature ramp (T-ramp) experiments. Chirascan systems allow for the simultaneous acquisition of multiparameter data, including CD, absorbance, fluorescence, and temperature. Both CD and fluorescence spectra as a function of temperature can be used to determine the melting temperature (Tm) of a protein via global fitting with Applied Photophysics’ Global3 software.

Image of Chirascan Q100 instrument with integrated autosampler.
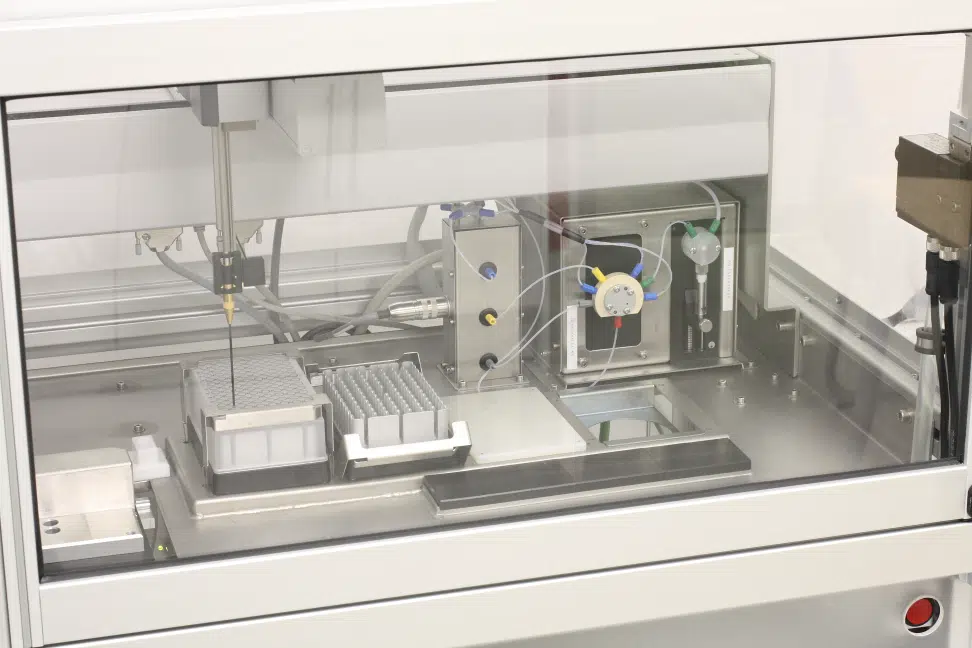
Closeup image of plate in autosampler.

High-Throughput Sample Analysis for HOS and T-Ramp Studies
The Chirascan Q100 is designed to run up to 96 samples per experiment in complete, unattended operation. This includes highly precise and reproducible sample handling and robust cleaning protocols, both prerequisites for statistically powerful HOS comparisons and time-consuming temperature ramp experiments. All samples and reference buffers are stored in temperature-controlled plates prior to analysis to ensure sample integrity throughout the experiment.
Chirascan platform accessories provide dedicated tools for specialized applications—for orthogonal data beyond circular dichroism, less common sample types, and additional measurement modes. Accessories are available for virtually every sample type and experiment, including:
- 6-Cell Turret – for increased sample throughput
- Titrator – for automated titrations
- pH Probe – for monitoring in-sample pH
- Solid Sample Holder – for measurements of solid (disc) samples
- Integrating Sphere – for measurements of powders and other opaque samples
- Stopped-Flow – for following kinetics by CD and other signal modes
- Circularly Polarized Luminescence (CPL) – for measuring the CD of luminescent compounds and calculating glum
- CCD Fluorometer – for fast, full emission spectrum fluorescence data
- Total Fluorescence – for detection of the total fluorescence emission as a function of excitation wavelength
- Scanning Emission Monochromator – for scanning the fluorescence emission wavelengths at a fixed excitation wavelength
- Couette Cell – for investigations of relative molecular orientation (Linear Dichroism)
- Optical Rotary Dispersion (ORD) – for measuring optical rotation/activity as a function of wavelength (Polarimetry)
- Magnetic Circular Dichroism (MCD) – for measuring the absorption of circularly polarized light by a sample in a magnetic field
Featured Capabilities / Case Studies – Courtesy of Applied Photophysics
Frequently Asked Questions
Learn more about Ichor’s CD services below: